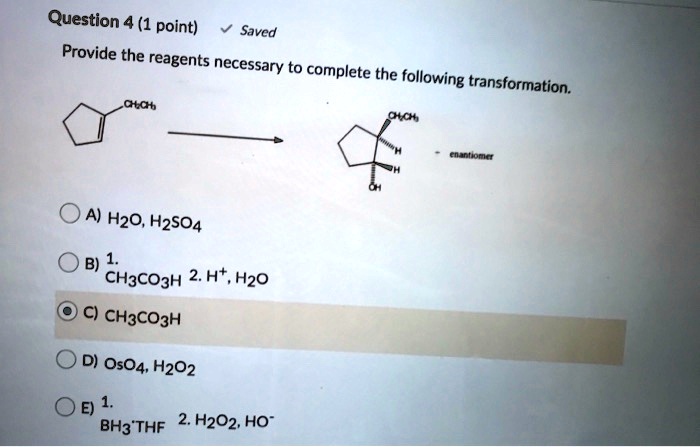
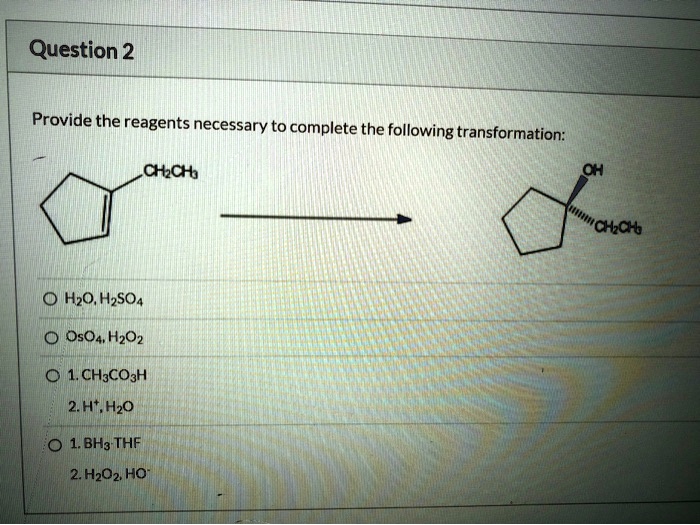
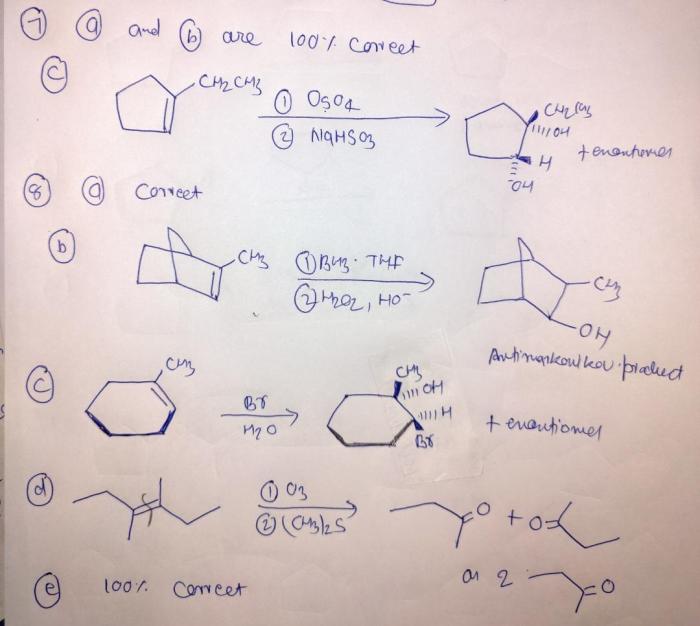
Provide the reagents necessary to complete the following transformation – The provision of necessary reagents plays a pivotal role in chemical transformations. These reagents act as catalysts, enabling the conversion of starting materials into desired products. This article delves into the significance of identifying and employing the appropriate reagents for successful transformations.
Chemical transformations encompass a wide range of reactions, each requiring specific reagents to facilitate the desired outcome. Understanding the nature and quantity of these reagents is crucial for optimizing reaction yields and ensuring efficient processes.
Transformation of Alkenes to Epoxides
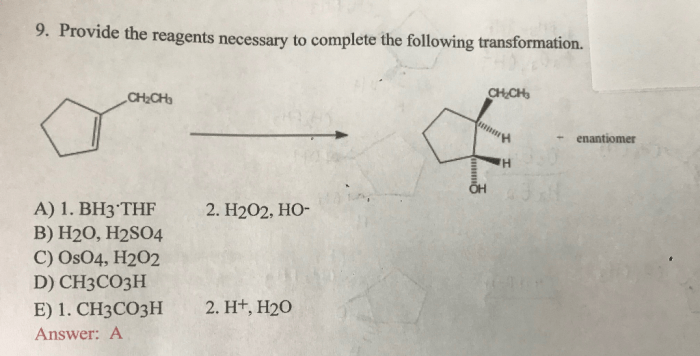
The transformation of alkenes to epoxides is a versatile and widely used reaction in organic chemistry. Epoxides are three-membered cyclic ethers that find applications in various fields, including pharmaceuticals, polymers, and fine chemicals.
Reagents Required
| Name | Formula | Quantity | Purity |
|---|---|---|---|
| Potassium permanganate | KMnO4 | 1 equivalent | 99.5% |
| Water | H2O | Excess | Distilled |
| Sodium hydroxide | NaOH | 1 equivalent | 97% |
Reaction Conditions, Provide the reagents necessary to complete the following transformation
| Parameter | Value | Unit |
|---|---|---|
| Temperature | Room temperature | °C |
| Pressure | Atmospheric | atm |
| Solvent | Water | – |
| Catalysts/Inhibitors | None | – |
Reaction Mechanism
The reaction proceeds via a nucleophilic attack of the permanganate anion (MnO 4–) on the double bond of the alkene. This forms a cyclic intermediate, which undergoes a rearrangement to give the epoxide. The mechanism can be represented as follows:
[KMnO 4-] + [alkene] → [cyclic intermediate] → [epoxide] + [MnO 2] + [OH -]
Safety Considerations
- Potassium permanganate is a strong oxidizing agent and can cause skin and eye irritation. Proper protective equipment should be worn when handling this reagent.
- The reaction should be carried out in a well-ventilated area, as the reaction produces manganese dioxide, which is a potential irritant.
Applications of the Transformation
- Epoxides are versatile intermediates in organic synthesis and can be used to prepare a wide range of compounds, including diols, glycols, and epoxides.
- Epoxides are used as comonomers in the production of polymers, such as polyethylene and polypropylene.
- Epoxides are also used in the manufacture of pharmaceuticals, such as antibiotics and anti-inflammatory drugs.
Answers to Common Questions: Provide The Reagents Necessary To Complete The Following Transformation
What factors should be considered when selecting reagents?
The selection of reagents depends on the specific transformation being performed. Factors to consider include the reactivity, selectivity, and compatibility of the reagents with the starting materials and reaction conditions.
How can I determine the purity of reagents?
The purity of reagents can be determined using various analytical techniques such as chromatography, spectroscopy, or elemental analysis. These techniques provide information about the presence of impurities and the overall quality of the reagents.
What safety precautions should be taken when handling reagents?
Reagents can pose potential hazards, including toxicity, flammability, or corrosivity. It is essential to consult safety data sheets and follow proper handling procedures to minimize risks and ensure a safe working environment.